You might wonder if aluminum oxide ionic or covalent. The answer is that aluminum oxide mostly has ionic bonding, but it shows covalent features too. Understanding its bonding helps you see why this compound acts differently from other metal oxides in chemistry and materials science.
Electronegativity plays a big role. Look at the difference between aluminum and oxygen in this table:
| Element | Electronegativity (Pauling scale) |
|---|---|
| Aluminum | 1.61 |
| Oxygen | 3.44 |
- Aluminum loses electrons and becomes Al³⁺.
- Oxygen gains electrons and becomes O²⁻.
- The electron transfer creates strong ionic bonds.
Polarization also matters. Aluminum oxide reacts with both acids and bases, which makes it special among metal oxides.
Key Takeaways
- Aluminum oxide primarily has ionic bonding, with some covalent characteristics due to electron sharing.
- The large difference in electronegativity between aluminum and oxygen leads to strong ionic bonds, making aluminum oxide stable and hard.
- Aluminum oxide’s unique properties, like high melting point and hardness, come from its mixed bonding type, useful in various industries.
- Understanding the bonding in aluminum oxide helps explain its behavior in applications like ceramics, electronics, and medical devices.
- Aluminum oxide’s amphoteric nature allows it to react with both acids and bases, setting it apart from other metal oxides.
Ionic vs Covalent Bonds

What Is Ionic Bonding
You see ionic bonding when one atom gives up electrons to another atom. This transfer creates charged particles called ions. Metals often lose electrons and become positive ions, while non-metals gain electrons and become negative ions. The opposite charges pull the ions together, forming a strong bond.
Ionic bonds usually form between metals and non-metals. These compounds often have high melting points and stay solid at room temperature.
Here is a simple table to help you compare ionic and covalent bonds:
| Property | Ionic Bonds | Covalent Bonds |
|---|---|---|
| Electron Interaction | Complete transfer of electrons | Sharing of electrons |
| Atom Types | Typically between metals and non-metals | Typically between non-metals |
| Resulting Particles | Formation of oppositely charged ions (cations and anions) | Formation of molecules with shared electron pairs |
What Is Covalent Bonding
Covalent bonding happens when two atoms share electrons. You find this type of bond mostly between non-metal atoms. The shared electrons help each atom reach a stable state. Covalent compounds can be solids, liquids, or gases at room temperature.
- Covalent bonds create molecules, not ions.
- These compounds often have lower melting and boiling points than ionic compounds.
- They do not conduct electricity in water.
Application to Aluminum Oxide
Now, let’s see how these ideas fit with aluminum oxide. In aluminum oxide, aluminum atoms transfer electrons to oxygen atoms. This process forms Al³⁺ and O²⁻ ions. The strong attraction between these ions creates a solid crystal with a high melting point and great hardness.
| Component | Quantity | Charge |
|---|---|---|
| Aluminum (Al) | 2 | +3 |
| Oxygen (O) | 3 | -2 |
You might ask, is aluminum oxide ionic or covalent? The answer is that it shows both types of bonding. The main bond is ionic, but some covalent character appears because of the way electrons interact between aluminum and oxygen. This mix of bonding types gives aluminum oxide its unique properties.
Aluminium Oxide Ionic or Covalent?
Ionic Nature of Aluminum Oxide
You can see that aluminum oxide has strong ionic bonds. Aluminum atoms lose electrons and become Al³⁺ ions. Oxygen atoms gain electrons and become O²⁻ ions. The attraction between these oppositely charged ions forms a solid crystal. This structure gives aluminum oxide its high melting point and hardness. When you ask, “is aluminum oxide ionic or covalent,” you notice that the ionic part stands out. The large difference in electronegativity between aluminum and oxygen supports this. Aluminum has an electronegativity of 1.61, while oxygen has 3.44. This gap means electrons move from aluminum to oxygen easily.
Ionic bonding makes aluminum oxide stable and tough. You find it in ceramics and as a protective coating because of these properties.
Covalent Characteristics in Aluminum Oxide
You also find covalent features in aluminum oxide. The electrons do not transfer completely. Some sharing happens between aluminum and oxygen atoms. This sharing creates covalent character in the bonds. Spectroscopic and computational studies show that aluminum oxide has mixed bonding. You see both ionic and covalent traits.
- The compound has a large band gap energy. This affects how it behaves in electronic devices.
- You find a low concentration of ionic defects and electronic carriers. This influences how aluminum oxide works at interfaces in technology.
When you look at aluminum oxide ionic or covalent, you see that covalent aspects help explain its unique behavior. The covalent character makes the bonds stronger and less likely to break apart in water.
Polarization and Bonding Type
Polarization plays a key role in the bonding of aluminum oxide. Aluminum ions are small and highly charged. They pull on the electrons of oxygen ions. This pull distorts the electron cloud around oxygen. You call this effect polarization. Polarization increases the covalent nature of the bond.
The question “is aluminum oxide ionic or covalent” depends on how much polarization occurs. More polarization means more covalent character.
You see that the bonding in aluminum oxide is not just one type. It is a mix. The ionic part comes from electron transfer. The covalent part comes from electron sharing and polarization. This combination explains why aluminum oxide acts differently from other metal oxides. You find it useful in many industries because of its special properties.
Evidence for Bonding Type
Electronegativity Difference
You can look at electronegativity to understand the type of bonding in aluminum oxide. Electronegativity measures how strongly an atom attracts electrons. When you compare aluminum and oxygen, you see a big difference:
- The electronegativity of aluminum is 1.61.
- The electronegativity of oxygen is 3.44.
- The difference between them is 1.83, which shows a strong ionic character.
This large gap means electrons move easily from aluminum to oxygen. Many other ionic compounds also have big electronegativity differences. This helps you answer the question: is aluminum oxide ionic or covalent? The numbers point to a mostly ionic bond.
Crystal Structure Insights
You can also learn about bonding by looking at the crystal structure. In aluminum oxide, aluminum atoms sit in both octahedral and tetrahedral sites. This means the atoms arrange themselves in two different ways. When you see this kind of structure, you know both ionic and covalent bonding are present. The special arrangement lets aluminum oxide have properties from both types of bonds. You find this mix in its hardness and stability.
The crystal structure of aluminum oxide shows that it is not just one type of bond. You see a blend of ionic and covalent features.
Scientific Observations
Scientists use different tools to study the bonds in aluminum oxide. They use X-ray diffraction, electron density maps, and other methods. These tools help you see where the electrons are and how the atoms connect.
| Methodology | Findings |
|---|---|
| Single-crystal X-ray diffraction | Weak residuals that look like bonding electrons appear. |
| Anharmonic refinement | Matches the known crystal structure. |
| Technique | Description |
|---|---|
| X-ray diffraction (XRD) | Shows the average structure of the crystal. |
| Quantitative convergent beam electron diffraction (QCBED) | Measures bonding effects at the nanoscale. |
You also find that computational studies show changes in electron distribution, especially at the metal-oxide interface. Some areas have less oxygen, which changes how electrons move. These results support the idea that aluminum oxide has both ionic and covalent character.
Bonding and Properties
Melting Point and Hardness
You notice that aluminum oxide stands out for its high melting point and impressive hardness. The strong ionic bonds between aluminum and oxygen atoms hold the crystal structure together tightly. This makes it difficult for the atoms to break apart, even at very high temperatures.
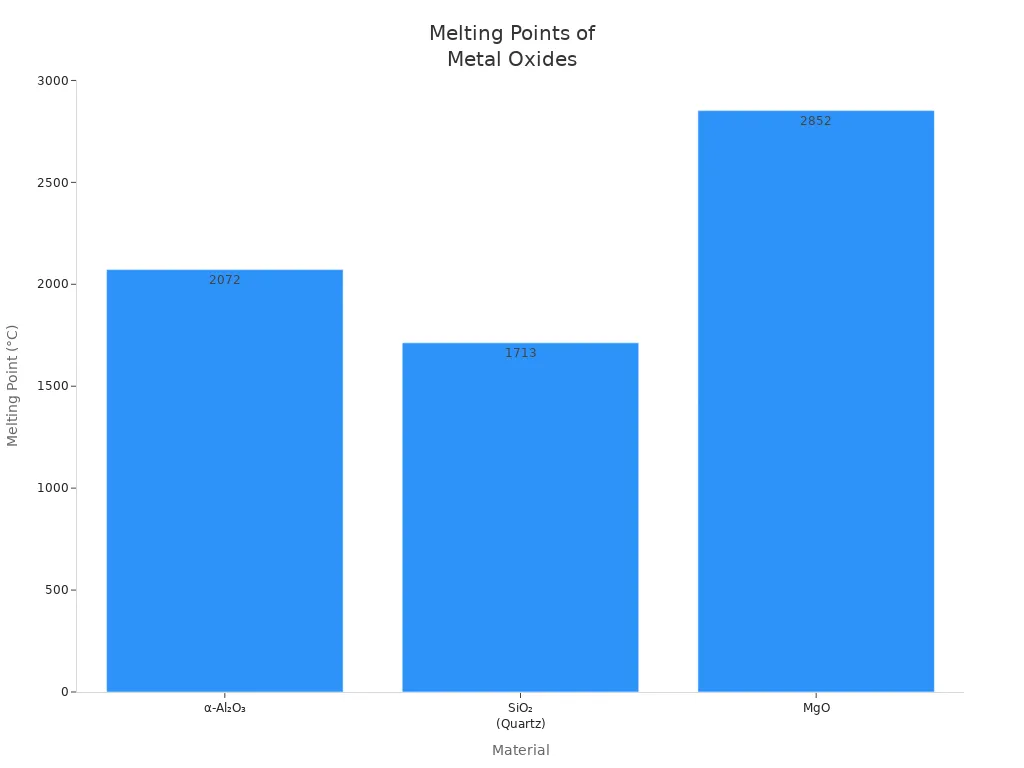
| Material | Crystal Structure | Melting Point (°C) | Notes |
|---|---|---|---|
| α-Al₂O₃ | Hexagonal | 2072 | Most stable form |
| SiO₂ | Trigonal | 1713 | Lower than alumina |
| MgO | Cubic | 2852 | Higher due to ionic bonds |
Aluminum oxide also ranks high on the Mohs hardness scale. You find it at a rating of 9, just below diamond. The close-packed arrangement of oxygen atoms with aluminum ions in octahedral spaces gives it this strength.
| Property | Value |
|---|---|
| Hardness | Mohs rating of 9 |
| Crystalline Structure | α-Al₂O₃ (Corundum) |
| Bonding Characteristics | Close-packed, durable |
Solubility and Reactivity
You see that aluminum oxide does not dissolve easily in water. The strong electrostatic forces between Al³⁺ and O²⁻ ions keep the compound together. This low solubility is typical for ionic compounds. However, aluminum oxide reacts with both acids and bases. You call this amphoteric behavior. The covalent character in its bonds allows these reactions.
| Property | Description |
|---|---|
| Ionic Nature | Electrostatic bonds between Al³⁺ and O²⁻ ions |
| Solubility in Water | Low, typical of ionic compounds |
| Amphoteric Nature | Reacts with acids and bases |
| High Melting Point | 2,072 °C, shows strong ionic bonding |
| High Thermal Conductivity | 35 W/mK, transfers energy efficiently |
Uses in Industry
You find aluminum oxide in many industries because of its unique bonding and properties. Its hardness makes it perfect for abrasives and cutting tools. The high melting point allows use in ceramics and refractories. The stable structure gives excellent electrical insulation for electronics. Medical and dental fields use it for implants because it is inert and biocompatible. You also see it as a catalyst support in chemical processing and as a scratch-resistant coating for glass and metals.
| Application | Contribution of Bonding Properties |
|---|---|
| Abrasives and Cutting Tools | Hardness makes it ideal for sandpaper and grinding wheels. |
| Refractories and Ceramics | High-temperature resistance due to strong ionic bonds. |
| Electronics and Semiconductors | Electrical insulating properties from its stable structure. |
| Medical and Dental Applications | Biocompatibility due to its inert nature. |
| Catalysts and Chemical Processing | Acts as a catalyst support due to its surface area. |
| Glass and Coatings | Scratch-resistant coatings due to hardness and stability. |
- Protective equipment (body armor, bulletproof windows)
- Electronics (circuit boards, semiconductors)
- Medical devices (dental implants, artificial joints)
- Catalysts in chemical processing (petrochemical refining)
- Scratch-resistant coatings for glass and metals
Metallization creates a surface on alumina that lets you bond it to metals using brazing. This transition layer helps overcome alumina’s resistance to direct bonding with metals.
You also benefit from aluminum oxide’s corrosion resistance. Anodized aluminum forms a tough oxide layer that protects against moisture and chemicals. The process is eco-friendly and does not produce hazardous waste.
| Consideration Type | Description |
|---|---|
| Corrosion Resistance | Anodized aluminum has a robust oxide layer that protects against moisture and chemicals. |
| Non-Toxic and Eco-Friendly | Safe for the environment, no hazardous waste produced. |
| Eco-Friendly and Sustainable | No VOCs emitted, sustainable choice. |
You now know that aluminum oxide has both ionic and covalent bonding. This mix explains its strength, stability, and many uses.
- You see it in ceramics, abrasives, and sunscreens because of its hardness and chemical resistance.
- Its bonding type also helps it work well in plastics and electronics.
| Finding | Implication |
|---|---|
| High thermal stability | Useful as a dielectric in electronics |
| Strong electrostatic interactions | Helps create better metal-polymer materials |
Recent studies show that the surface structure of aluminum oxide affects its properties. This knowledge helps scientists design better materials for the future.
FAQ
What makes aluminum oxide different from other metal oxides?
You see aluminum oxide act as both an acid and a base. This amphoteric behavior sets it apart from most metal oxides. Its mixed ionic and covalent bonding gives it unique chemical properties.
Why does aluminum oxide have such a high melting point?
You find strong ionic bonds between aluminum and oxygen ions. These bonds hold the atoms tightly together. The crystal structure resists heat, so aluminum oxide melts at 2,072°C.
Tip: High melting points often mean strong bonds in the crystal.
Can aluminum oxide conduct electricity?
You notice aluminum oxide does not conduct electricity. The ions stay locked in place within the crystal. No free electrons or ions move, so it acts as an electrical insulator.
| Property | Aluminum Oxide |
|---|---|
| Conductivity | No |
| Insulator | Yes |
Where do you use aluminum oxide in daily life?
You use aluminum oxide in many products. It appears in sandpaper, ceramics, medical implants, and scratch-resistant coatings. Its hardness and stability make it valuable in these applications.
- Sandpaper
- Ceramics
- Medical implants
- Coatings