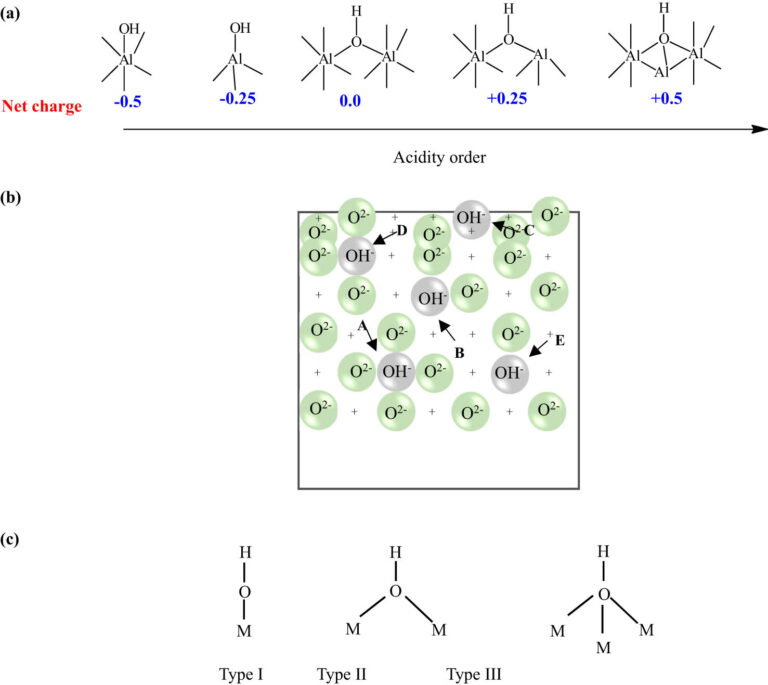
Aluminum oxide, commonly known as alumina, is a vital material in various industries. Its unique properties make it indispensable in fields like electronics, ceramics, and refractories.
Understanding its crystal structure is crucial for optimizing its applications. The most stable crystalline form of aluminum oxide is corundum.
Corundum is renowned for its hardness and high melting point. This makes it an excellent abrasive material.
Alumina exists in several other crystalline forms, each with distinct properties. These include gamma, delta, and theta alumina.
The crystal structure of alumina is characterized by a hexagonal close-packed arrangement. This arrangement influences its thermal and mechanical properties.
Advances in technology have enhanced our understanding of alumina’s crystal structure. Techniques like X-ray diffraction and electron microscopy play a significant role.
Research continues to explore new applications for alumina’s crystal forms. This ongoing study is vital for developing advanced materials.
Understanding the crystal form of aluminum oxide is essential for students, researchers, and professionals in materials science.
What Is Aluminum Oxide? An Overview
Aluminum oxide, with the chemical formula Al2O3, is a significant inorganic compound. It is commonly referred to as alumina. This compound is critical in both science and industry.
Alumina is composed of aluminum and oxygen. It occurs naturally as the mineral corundum. This mineral includes gemstones like sapphires and rubies.
Let’s explore some basic facts about aluminum oxide:
- Chemical Formula: Al2O3
- Natural Form: Corundum
- Gemstone Varieties: Sapphires and rubies
- Common Name: Alumina

This compound is crucial for various applications due to its properties. It is used in abrasive materials, refractory products, and ceramics. Alumina’s versatility is due to its stability and resistance to chemical attack.
The presence of alumina in different crystal forms affects its properties. Gamma, delta, and theta are other forms besides corundum. Each form has unique characteristics making them suited for specific uses. Understanding these forms is essential for effectively utilizing aluminum oxide in various technologies.
Chemical and Physical Properties of Alumina
Alumina exhibits a range of distinctive chemical and physical properties. Its chemical stability is one of its most notable traits. This stability makes alumina resistant to many chemicals, including acids and bases.
Physically, aluminum oxide is known for its remarkable hardness. This characteristic makes it an excellent abrasive material. Corundum, its most stable form, is harder than most natural substances.
Let’s delve into the key properties of alumina:
- Chemical Stability: Resistant to acids and bases
- Hardness: High, making it an effective abrasive
- Melting Point: Approximately 2,072°C
- Density: About 3.95 g/cm³

Alumina also displays excellent thermal conductivity. This property enables it to effectively dissipate heat. Moreover, it acts as a superb electrical insulator.
High thermal and chemical resistance makes alumina ideal for harsh environments. Industries utilize these properties for manufacturing refractories and protective coatings. Thus, alumina’s properties contribute significantly to its versatility and applicability in diverse fields. Understanding these characteristics is fundamental for optimizing alumina’s use in various industrial applications.
Crystalline Forms of Aluminum Oxide
Aluminum oxide, commonly known as alumina, boasts several crystalline forms. Each form has unique properties and applications. Understanding these forms is essential for their effective use.
The most recognized crystalline form of aluminum oxide is corundum. It’s highly stable and extensively used in various industries. Corundum’s robustness and high melting point make it invaluable.
Beyond corundum, alumina exists in metastable phases. These include gamma, delta, and theta alumina. Each phase serves different purposes in industrial applications.
Key forms of aluminum oxide include:
- Corundum: Most stable, used as gemstones like sapphires
- Gamma Alumina: High surface area, ideal for catalyst support
- Delta and Theta Alumina: Serve unique roles in thermal management

by Albert Hyseni (https://unsplash.com/@alberthyseni)
The transformation of these phases depends on conditions like temperature and pressure. Such transformations can significantly affect alumina’s performance. Therefore, controlling synthesis conditions is crucial.
Studying the crystalline forms of aluminum oxide aids in harnessing their full potential. Knowledge of these forms helps in the development of advanced materials.
Corundum: The Most Stable Crystal Form
Corundum stands out as the most stable form of aluminum oxide. It is well-known for its hardness, ranking just below diamond. This makes corundum an essential material in abrasive applications.
In nature, corundum forms gemstones, such as sapphires and rubies. These gemstones owe their colors to trace impurities within the crystal structure. The presence of iron, titanium, or chromium alters the stone’s hue.
Listed characteristics of corundum include:
- Hardness: Second only to diamond on the Mohs scale
- Optical Properties: Used in lasers and specialized optics
- Thermal Stability: Withstands high temperatures without degrading
by Susan Wilkinson (https://unsplash.com/@susan_wilkinson)
Industrially, corundum serves as a robust abrasive in machining. Its durability makes it perfect for cutting, grinding, and polishing. Additionally, it is used in high-temperature refractory applications.
The crystalline structure of corundum is a trigonal arrangement. This structure contributes to its strength and hardness. The atomic arrangement also plays a role in its thermal and optical properties.
Other Crystalline Forms: Gamma, Delta, and Theta Alumina
Besides corundum, alumina has other crystalline forms. These metastable phases are gamma, delta, and theta alumina. Each phase possesses distinct characteristics and potential uses.
Gamma alumina is particularly notable for its high surface area. This property makes it ideal for use in catalyst supports. Its structure allows for effective absorption and reaction facilitation.
Key characteristics of these metastable forms include:
- Gamma Alumina: Optimal for catalysis due to high surface area
- Delta Alumina: Intermediate phase in temperature-induced transitions
- Theta Alumina: Transforms into alpha alumina at high temperatures
by Steve Johnson (https://unsplash.com/@steve_j)
Transformation between these phases occurs under specific conditions. Temperature and pressure play crucial roles in these changes. Understanding these transitions is vital for tailoring material properties.
Each form serves distinct roles in advanced materials. Continued research explores their application in nanotechnology and electronics. This ongoing study promises to further unlock their potential.
The Al2O3 Crystal Structure in Detail
The Al2O3 crystal structure is both intricate and fascinating. Understanding this structure is crucial for its application in various fields. Alumina’s arrangement affects its thermal and mechanical properties significantly.
At the atomic level, alumina forms through a specific geometric layout. Oxygen ions create a hexagonal close-packed structure. Aluminum ions fill two-thirds of the octahedral spaces within this arrangement.
This unique packing results in several benefits:
- High Melting Point: Perfect for high-temperature applications
- Electrical Insulation: Ideal in electronics and circuitry
- Thermal Conductivity: Efficient in heat management
by Mehdi Mirzaie (https://unsplash.com/@mirzaie)
Al2O3’s structure contributes to its robust nature. The strong ionic bonds give alumina considerable hardness. These bonds also enable chemical stability under extreme conditions.
The intricacy of alumina’s lattice is a central focus of materials science. Advances in technology have allowed for deeper insights into this structure. Tools like X-ray diffraction reveal details previously unseen.
Interest in Al2O3’s crystal form continues to grow. Researchers aim to tailor its properties further. This pursuit enhances alumina’s utility in cutting-edge technologies.
Corundum Crystal Structure: Atomic Arrangement
Corundum, the most recognized form of alumina, showcases a distinct atomic arrangement. It has a trigonal structure, which defines its strength. This configuration is due to the specific positioning of atoms within the lattice.
In corundum, oxygen ions form layers, creating a dense pack. Aluminum ions fit snugly into these layers, optimizing space. This tight configuration gives corundum its characteristic hardness.
Primary attributes of the atomic arrangement include:
- Trigonality: Results in exceptional strength
- Density: Contributes to hardness and durability
- Symmetry: Promotes uniform physical properties

by Rick Rothenberg (https://unsplash.com/@rick_rothenberg)
The trigonal structure impacts how light and heat interact with corundum. Its optical clarity finds uses in optical devices and gemstones. Meanwhile, the dense atomic packing aids in thermal resistance.
Corundum’s atomic layout is pivotal in high-performance materials. Engineers and scientists leverage its properties for industrial applications. Its study helps improve methods for material synthesis and modification.
How Crystal Structure Influences Properties
The crystal structure directly impacts aluminum oxide’s properties. The atomic arrangement within the crystal affects its overall behavior. These effects determine how alumina can be used effectively.
Several properties are influenced by the crystal lattice:
- Mechanical Strength: High due to robust atomic packing
- Electrical and Thermal Conductivity: Affected by lattice arrangement
- Chemical Reactivity: Limited by strong ionic bonds
by asia Dh (https://unsplash.com/@as_dh)
Mechanical strength stems from the tight atomic configuration. This makes alumina resistant to physical wear and damage. Its hardness comes from the rigidity of its ionic lattice.
The electrical and thermal characteristics depend on the energy movement through the crystal. Alumina’s structure acts as an insulator in electronics. This is due to the dense configuration preventing free electron flow.
Chemical resistance is another critical property. The structure’s bonds don’t easily break under chemical exposure. This stability makes alumina suitable for corrosive environments.
Understanding these correlations is crucial for designing applications. Materials scientists study crystal structures to harness such properties. This knowledge expands alumina’s use in advanced technologies.
Methods for Studying Alumina Crystals
Studying the crystal structure of alumina involves sophisticated techniques. These methods uncover the intricate details of its atomic arrangement. Each technique offers unique insights that are vital for research.
Common methods include:
- X-ray Diffraction: Analyzes spacing between atomic planes
- Electron Microscopy: Provides images of atomic arrangement
- Neutron Scattering: Investigates magnetic properties at the atomic scale
by Andrei Castanha (https://unsplash.com/@andreicastanha)
X-ray diffraction remains a cornerstone technique. It allows for precise measurements of crystal lattices. This method is essential for identifying various crystal forms.
Electron microscopy complements this by offering high-resolution images. Researchers can visualize and interpret the atomic landscape of alumina. This visual data aids in a deeper understanding of its structural nuances.
Through these advanced techniques, we can explore alumina in detail. Continuous advancements in technology enhance these methods, leading to new discoveries in the field.
Industrial and Technological Applications of Alumina Crystals
Alumina crystals have broad applications in various industries due to their unique properties. Their versatility is seen in both traditional and high-tech applications.
One major area of use is in the production of ceramics. Alumina’s high hardness and thermal stability make it ideal for robust ceramic components. These components are vital in many industrial processes.
In the electronics sector, alumina plays a crucial role. It is used in electronic substrates and insulators. This is largely due to its excellent electrical insulation and thermal conductivity.

by Y M (https://unsplash.com/@ymoran)
Additionally, alumina finds application in laser technology. Corundum, a crystalline form, is used in laser crystals, enhancing their optical properties. This is critical for devices requiring precision and efficiency.
Alumina also supports the catalytic industry:
- Catalyst support: Enhances reaction rates and efficiency
- Abrasion-resistant materials: Used in mining tools and wear-resistant parts
These diverse applications underscore alumina’s industrial significance. As technology advances, the demand for high-performance materials like alumina continues to grow, spurring ongoing research and development.
Advances in Research and Future Directions
Research into alumina crystals is evolving rapidly. Scientists explore novel synthesis methods to enhance its properties. These efforts aim to optimize alumina’s applications in emerging technologies.
Recent studies focus on the nano-scale. Nanostructured alumina promises improved mechanical strength and flexibility. This opens doors for its use in cutting-edge fields like nanotechnology and biotechnology.
Future research directions include:
- Energy storage: Enhancing battery efficiency and capacity
- Biomaterials: Developing biocompatible implants and prosthetics
- Photovoltaics: Improving the efficiency of solar cells
Continuous advancements will likely foster new applications. This will further cement alumina’s role in next-generation materials. The future holds immense potential for alumina crystals in transforming technology landscapes.
Frequently Asked Questions About the Crystal Form of Aluminum Oxide
What is alumina’s most stable form?
The most stable form is corundum. It is appreciated for its hardness and thermal stability.
How does temperature affect alumina’s structure?
Temperature can cause phase transitions in alumina. This changes the structure from one crystalline form to another.
Why is alumina important in industries?
Alumina’s properties, such as abrasion resistance and electrical insulation, make it valuable in various industrial applications.
Is alumina used in electronics?
Yes, alumina serves as a substrate due to its excellent thermal conductivity. It is fundamental in electronic circuits.
List of alumina’s applications:
- Ceramics
- Refractories
- Catalyst supports
- Abrasives
Conclusion: The Importance of Understanding Alumina’s Crystal Structure
The study of alumina’s crystal structure is crucial for advancing materials science. By understanding its unique properties, we can optimize its uses across various industries.
Exploring alumina’s crystalline forms unlocks possibilities for new applications. Continued research will keep alumina at the forefront of technological innovation and material development.