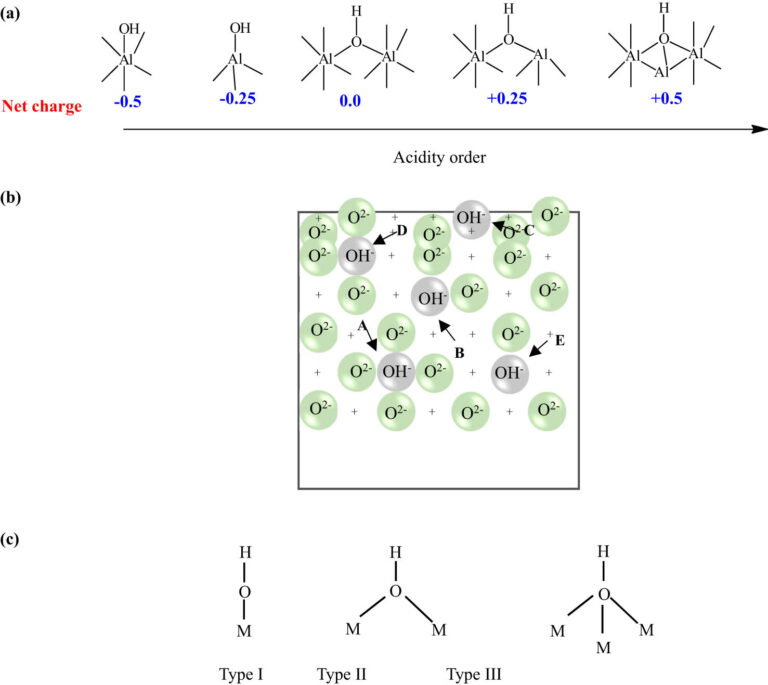
Al2O3, commonly known as alumina, is a vital ceramic material. It is renowned for its exceptional thermal and electrical properties.
Understanding these properties is crucial for various applications. One key property is the thermal expansion coefficient of Al2O3.
This coefficient measures how much alumina expands when heated. It is essential for applications involving temperature changes.
Alumina’s thermal expansion coefficient is relatively low. This makes it ideal for high-temperature environments.
Another important property is alumina’s specific heat capacity. This determines how much heat the material can absorb and release.
Al2O3 also has a significant dielectric constant. This affects its performance in electronic and electrical applications.
These properties make alumina a versatile material. It is used in industries ranging from aerospace to electronics.
In this article, we will explore these thermal properties in detail. Understanding them is key to leveraging alumina’s full potential.
What is Al2O3 (Alumina)?
Al2O3, or alumina, is a chemical compound composed of aluminum and oxygen. It is one of the most abundant and naturally occurring compounds on Earth.
Alumina is widely recognized for its role as a primary component in bauxite ore, the principal source of aluminum metal. However, beyond this, alumina has many other industrial uses.
Alumina comes in various forms and grades, reflecting its diverse applications. Its forms include both crystalline and amorphous, with properties that influence its use.

One notable crystalline form is corundum, which is a part of rubies and sapphires. This form is prized for its hardness and color.
Some key properties of alumina include:
- High melting point
- Chemical inertness
- Electrical insulation
- Hardness and strength
Its unique properties make it ideal for various applications, including ceramics, refractories, abrasives, and cutting tools. Understanding alumina’s structure and properties is essential for utilizing it effectively in advanced materials and engineering projects. By leveraging these characteristics, industries can design products that endure extreme conditions and excel in performance.
The Importance of Thermal Expansion in Materials
Thermal expansion is a fundamental material property. It describes how a material changes size as its temperature varies. This expansion or contraction affects nearly all substances.
In practice, understanding thermal expansion is crucial for material selection. Components must fit and function correctly under temperature changes. Ignoring this can lead to failures or inefficiency.
Materials expand at different rates. Some expand quickly with heat, while others change very little. These differences play a critical role in material compatibility and engineering design.
A few key reasons thermal expansion matters include:
- Maintaining tight tolerances
- Preventing structural failure
- Ensuring mechanical stability
- Enhancing thermal management
For engineering applications, precise calculations and knowledge of thermal expansion prevent costly mistakes. It helps in creating products that resist cracking or warping under thermal stress. When selecting materials, designers consider thermal expansion coefficients to pair materials that behave predictably together. This ensures longevity and performance in real-world conditions. By understanding and managing thermal expansion, industries achieve safer and more reliable products.
Thermal Expansion Coefficient of Al2O3: Key Facts and Values
Al2O3, commonly known as alumina, is a widely used ceramic. Its popularity stems from its superior thermal and electrical properties. One crucial characteristic of alumina is its thermal expansion coefficient.
The thermal expansion coefficient measures how much a material expands with temperature change. For Al2O3, this coefficient is approximately 8.5 x 10^-6 /°C at room temperature. This low value is advantageous in various high-temperature applications.
Compared to metals, alumina’s thermal expansion coefficient is significantly lower. This low expansion rate minimizes the risk of cracking under thermal stress. It makes Al2O3 especially valuable in environments with rapid temperature fluctuations.
It’s important to note that the thermal expansion of Al2O3 is anisotropic. This means it can vary in different crystallographic directions. Such property nuances require careful consideration when designing components with alumina.
Understanding the thermal expansion behavior of Al2O3 is key. Engineers leverage this knowledge in designing structures exposed to thermal cycling. Compatibility with other materials in assemblies is ensured by this understanding.
Here’s a summary of key thermal expansion facts for Al2O3:
- Low thermal expansion coefficient of about 8.5 x 10^-6 /°C
- Anisotropic expansion behavior
- Excellent dimensional stability in thermal cycling applications
Alumina’s thermal properties make it suitable for complex applications. It contributes to precision in manufacturing and assembly tasks. By understanding these properties, industries maximize the performance and reliability of alumina-based products.
The crystalline structure and bonding within Al2O3 largely influence its expansion behavior. This microscopic arrangement dictates how atoms within the material react to heat. Factors like purity and microstructure further modify this expansion coefficient.
This attention to detail ensures that Al2O3 remains a trusted choice in high-performance scenarios. Its predictable behavior under thermal stress offers confidence to engineers and designers. With a deep understanding of its properties, Al2O3 continues to excel across various industries.
Factors Affecting the Thermal Expansion of Al2O3
Several factors influence the thermal expansion of Al2O3. Understanding these factors helps in predicting and optimizing its behavior in various applications. Variability in expansion can arise from inherent material properties and external conditions.
The purity of Al2O3 is a significant determinant. Impurities within the material can alter the thermal expansion coefficient. These impurities can introduce different phases, affecting the uniformity of expansion.
Microstructure also plays a critical role. The arrangement and size of grains in the material impact its expansion. Finer grains typically result in a more uniform response to thermal changes.
External factors such as temperature range and cycling impact expansion. Al2O3 exhibits slight increases in its thermal expansion coefficient at elevated temperatures. Thus, temperature fluctuations require careful consideration.
Here’s a concise list of influencing factors:
- Purity: Presence of impurities and different phases
- Microstructure: Grain size and arrangement
- Temperature conditions: Fluctuations and ranges
Understanding these factors allows engineers to tailor Al2O3 for specific needs. Adjustments to purity and microstructural features enhance performance. Such modifications ensure materials withstand the demanding conditions of various applications.
by Mathew Schwartz (https://unsplash.com/@cadop)
By managing these variables, Al2O3 can be optimized for high-temperature environments. This precision boosts its effectiveness in complex industrial tasks. It remains a preferred choice for engineers seeking reliable performance.
Comparison: Alumina vs. Other Materials
Al2O3, or alumina, stands out for its thermal properties. Its thermal expansion coefficient is lower than many metals. This makes it a leading choice for high-temperature applications where thermal stability is essential.
When compared to metals like aluminum and steel, alumina displays a reduced thermal expansion. Metals typically expand more with temperature changes, risking structural integrity. Alumina’s stability provides a significant advantage in maintaining dimensional accuracy.
In contrast with other ceramics, Al2O3 often exhibits moderate expansion properties. While ceramics like silicon carbide might have even lower thermal expansion, alumina offers a balance of insulating properties and mechanical strength.
Here’s how Al2O3 compares with various materials:
- Metals: Lower expansion, better thermal stability
- Ceramics (e.g., silicon carbide): Moderately higher expansion, better balance
- Glasses: Lower expansion, more mechanical and thermal durability
Alumina’s competitors in thermal management include polymers and composite materials. Although polymers can offer low thermal expansion, they lack alumina’s thermal resilience and mechanical durability.

by Kelly Sikkema (https://unsplash.com/@kellysikkema)
Overall, Al2O3’s unique combination of properties makes it valuable in applications where both thermal and mechanical performance are vital. Its use in high-precision instruments exemplifies its critical role in industrial and technological fields.
Alumina Specific Heat Capacity and Its Role in Thermal Management
Alumina’s specific heat capacity plays a crucial role in its thermal management applications. This property indicates how much heat the material can absorb before its temperature rises significantly.
The specific heat capacity of alumina is approximately 0.9 J/g°C. This is relatively high compared to other ceramics, allowing it to effectively absorb and moderate heat. This characteristic is vital in scenarios requiring thermal stability under fluctuating temperatures.
In thermal management, materials with higher specific heat are desirable. They act as heat buffers, protecting components from rapid temperature shifts. This makes alumina an excellent choice for electronic devices where overheating is a concern.
Key aspects related to alumina’s heat capacity include:
- Heat Absorption: Manage temperature changes effectively
- Energy Storage: Reduces temperature spikes
- Thermal Stability: Supports steady performance
This specific heat capacity means alumina can maintain its properties across a range of temperatures. For applications like heat exchangers, this stability is invaluable, preventing hot spots and extending the lifespan of components.

by Laura Jaeger (https://unsplash.com/@lvjart)
Beyond thermal buffering, the specific heat affects how alumina dissipates heat. In high-heat environments, this ability prevents thermal fatigue, a common cause of failure in lesser materials. As industries push for improved thermal management, alumina remains a preferred option. Its performance in energy-intensive applications underscores its importance in modern engineering challenges.
Al2O3 Dielectric Constant: Electrical and Thermal Interplay
Alumina’s dielectric constant is a vital property influencing its use in electrical applications. With a value around 9.8, it effectively supports electrical insulation while maintaining heat resistance.
This balance of electrical and thermal properties makes Al2O3 highly valued in electronics. It serves as an insulator in capacitors, ensuring efficient performance under demanding conditions. The dielectric constant directly impacts the material’s ability to store and release electrical energy.
Factors affecting the dielectric constant include:
- Purity: Higher purity leads to better insulating properties.
- Temperature: Changes in temperature can slightly alter the dielectric properties.
- Frequency: Electrical frequency can influence dielectric response.
The integration of Al2O3 in devices depends largely on these properties. Its ability to withstand electrical stress without losing thermal integrity offers engineers a reliable material for innovative designs.

by Julia Maior (https://unsplash.com/@juliamaior)
In circuits where both thermal and electrical stability are needed, alumina excels. This dual capability supports its use in modern technologies that demand efficiency and durability. As technology evolves, the interplay of these properties ensures alumina remains central in advanced electrical applications.
Applications of Al2O3 Based on Its Thermal Properties
Al2O3, or alumina, is utilized in numerous applications, primarily due to its exceptional thermal properties. Its low thermal expansion coefficient and high melting point make it ideal for environments with fluctuating temperatures.
In the aerospace industry, alumina is crucial for components that endure extreme thermal cycling. Its ability to maintain structural integrity at high temperatures ensures safety and efficiency in flight operations.
The automotive sector also benefits from alumina’s thermal stability. It is used in engine parts and exhaust systems, where thermal management is essential for optimal performance and longevity.
Electronic devices employ alumina as a substrate material due to its excellent thermal conductivity. This property helps dissipate heat efficiently, protecting sensitive electronic components from overheating.
Key applications of Al2O3 include:
- Aerospace Components: High thermal stability is essential.
- Automotive Parts: Key in engine and exhaust systems.
- Electronics: Used in substrates and insulating materials.
- Refractory Materials: Withstands high temperatures.
- Chemical Processing Equipment: Resists thermal and chemical stress.

by Bekky Bekks (https://unsplash.com/@bekkybekks)
In chemical processing, alumina’s thermal properties allow it to withstand harsh environments without degrading, making it indispensable for containers and liners. Due to its versatile thermal properties, alumina continues to play a pivotal role in advanced and everyday technologies, driving innovation across multiple industries.
Engineering Considerations: Using Al2O3 in High-Temperature Environments
When designing components for high-temperature environments, engineers must account for the thermal expansion properties of materials. Al2O3, with its low thermal expansion coefficient, is a popular choice.
Incorporating Al2O3 into designs reduces the risk of thermal stresses, which could lead to component failure. This stability is crucial in systems where temperature fluctuations are common.
Engineers also consider compatibility when using Al2O3 alongside other materials. Its expansion rate should align closely with those of adjacent materials to prevent misalignment or cracking.
Design considerations include:
- Thermal Stress Management: Ensuring structural integrity under thermal cycling.
- Material Compatibility: Matching expansion rates with other materials.
- Maintenance Costs: Reducing wear over time increases longevity.
- Safety Requirements: Ensuring reliability in critical components.
Careful planning and comprehensive testing of Al2O3 applications in high-temperature settings can lead to improved system performance. By fully understanding its thermal characteristics, engineers can create efficient and resilient systems for demanding applications.
Summary Table: Key Thermal and Electrical Properties of Al2O3
Al2O3 exhibits several important thermal and electrical properties that make it a material of choice in demanding applications. Its specific heat capacity and dielectric constant complement its low thermal expansion coefficient.
Below is a summary table highlighting these key attributes of Al2O3, offering a quick reference for engineers and materials scientists:
| Property | Value | |———————————|—————————| | Thermal Expansion Coefficient | ~8.5 x 10^-6 /°C | | Specific Heat Capacity | ~0.9 J/g°C | | Dielectric Constant | ~9.8 |
This table serves as a concise guide to understanding Al2O3’s performance in thermal and electrical contexts. These properties underscore its versatility in various industrial applications.
Conclusion: The Value of Understanding Alumina’s Thermal Expansion
Grasping the thermal expansion properties of Al2O3 is crucial for its effective use in modern industries. This understanding ensures that alumina is correctly applied in environments where temperature stability and reliability are paramount.
Al2O3’s unique thermal attributes, including its low expansion rate, make it ideal for high-temperature uses. These properties allow engineers to design components that withstand thermal stresses, reducing risks of material failure.
As technology and industry demands grow, the significance of Al2O3’s thermal behavior becomes even more evident. Knowing these properties enables innovation, offering solutions that efficiently meet the challenges of diverse sectors. Ultimately, insight into Al2O3’s thermal characteristics supports advancements in material science and engineering.